Alternative form of SFPQ protein could drive ALS
This represents a potential therapeutic target, which could ultimately alleviate damage to nerve cells.
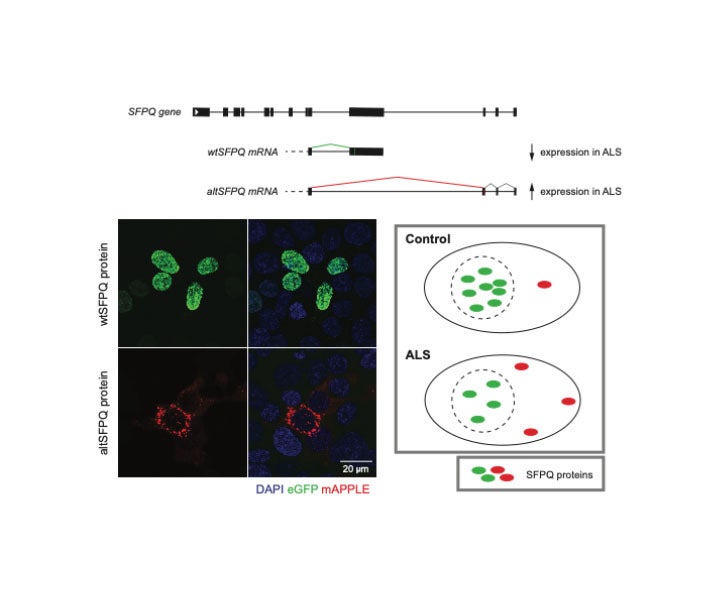
Motor neuron disease or ‘ALS’ is a truly devastating condition for which the need for impactful therapy is apparent and urgent. Whilst over 40 ALS-causative gene mutations have been identified most patients, in fact, present without any such known mutations. This poses a critical knowledge void regarding how such molecular diversity can manifest in common clinical symptoms. Importantly, however, particular pathologies are common across the majority of ALS patients. Specifically, a small number of proteins involved in orchestrating RNA molecules, are found misplaced within ALS patient nerve cells.
Many RNAs are incorrectly governed within the context of ALS, with direct contribution to ALS nerve cell damage. Therefore, understanding what drives this perturbed protein and RNA ‘governance’ is vital to pinpoint potential therapeutic targets upstream within the disease process. Our paper specifically addresses this question for one such protein, SFPQ. We reveal a new version (‘isoform’) of the SFPQ protein which possesses inherently different localisation within the cell compared to the regular SFPQ protein, and report how this is regulated at the molecular level.
Crucially, we show in various ALS models that there is a shift in SFPQ usage / production away from the regular form, and towards this new ‘alternative’ version of SFPQ, therefore providing a potential explanation for the abnormal location of SFPQ observed in ALS patient tissues. Further still, we characterise the behaviour and function of this novel protein, providing greater insight into its potential importance in ALS-affected cells. Altogether, our study provides a new and targetable mechanism for the correction of SFPQ levels / localisation, which may alleviate some of the negative downstream consequences for RNA molecules, and ultimately damage to nerve cells in ALS.
You can read more on the publication here.

