Mission Statement
1. To conduct independent reviews of the ethical merits of research projects that involve human subjects or human tissues, proposed by academic NUS staff.
2. To approve all relevant projects, in accordance with the Good Clinical Practice (GCP) and Bioethics Advisory Committee (BAC) guidelines and the applicable laws and regulations in Singapore that govern or regulate research involving human subjects.
3. To monitor research projects, by way of regular reports, as determined by the NUS-IRB.
4. To submit an annual report, and such other reports, as may be called for by the President of NUS, or any other relevant authority.
Research studies that do not require ethic clearance from NUS-IRB include:
(i) studies involving only literature review or;
(ii) studies that do not recruit human participants, use human biological materials/human tissue or human data or;
(iii) studies that involve only animals.
For any doubts or queries, please contact
NUS-IRB.
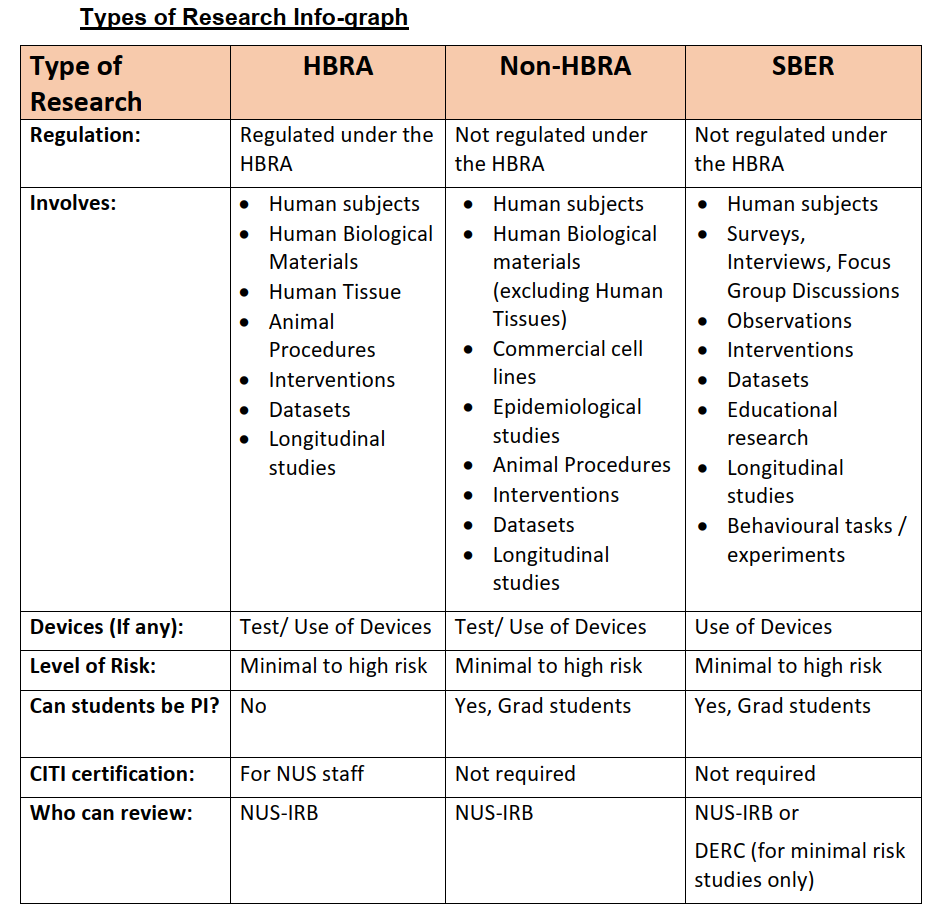
In accordance to the University's guidelines, all NUS human subjects research studies must be reviewed by either the NUS Institutional Review Board (NUS-IRB) or the Department Ethics Review Committee (DERC).
The purposes of ethics review by the NUS-IRB and DERCs:- - to ensure the methods proposed for research are ethical for the protection of the rights, safety and welfare of human research participants.
- to represent the interests of (protect) research participants by ensuring the project has purpose, is as safe as it can be and that any possible risk of harm is both minimized and managed.
- to represent the interests of (protect) participants and public by ensuring the project has a clear question it proposes to answer, that the chosen method has a reasonable chance to do this and answers will be both meaningful and valid.
- to ensure that anyone thinking of participating in a study is given a fair opportunity to accept or decline.
- to ensure that the research complies with national legislation.
Definition of Minimal Risks
A risk is minimal where the probability and magnitude of harm or discomfort anticipated in the research are not greater, in and of themselves, than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.
|
ac637a9187704605ab13a0ddcea3d0a6.jpg?sfvrsn=8b133c32_0)
| For any queries, please contact NUS-IRB or DERC. Faculty and student SBER that qualifies for an Exemption that is of minimal risk may be reviewed and approved by a DERC. Excludes any HBR falling within section 3 of the HBRA.
|
What is a DERC?
A DERC is a committee formed at the school, faculty or department level to perform ethics review on research studies. Faculty research studies that qualify for an Exemption review and student research studies that qualify for an Exemption or Expedited review may be reviewed and approved by a DERC. DERC may refer the studies to the NUS-IRB for review where necessary.
For more info on DERC, click here
|
IRB - Institutional Review Board
PI - Principal Investigator
Service quality timelines: The IRB Secretariat aims to have a turn-around time of 10 working days for SBER exempted reviews, 30 working days for expedited and full reviews, and 20 working days for protocol amendments.
For any queries, please contact NUS-IRB or DERC

